The world of organic chemistry is vast and intricate, filled with molecules that shape our daily lives in countless ways. Among the most fundamental and fascinating of these molecular architectures are arenes, a class of hydrocarbons characterized by their unique aromaticity. From the fuels that power our vehicles to the pharmaceuticals that heal our bodies, arenes form the backbone of an astonishing array of essential substances. Understanding these compounds is not merely an academic exercise; it's a journey into the very heart of modern chemistry and its profound impact on industry, medicine, and technology.
This comprehensive exploration will demystify arenes, delving into their distinctive structure, diverse derivatives, and the intriguing reactions they undergo. We will uncover why these compounds are so special, how they are identified, and their pervasive influence across various sectors. Prepare to embark on a detailed scientific voyage that illuminates the foundational role of arenes in the chemical landscape.
Table of Contents
- What Exactly Are Arenes? Defining Aromaticity
- The Unique Structure of Arenes: Benzene as the Blueprint
- Common Arenes and Their Derivatives
- Reactivity of Arenes: Electrophilic Aromatic Substitution (EAS)
- Arene Spectroscopy: Unveiling Molecular Fingerprints
- Industrial Importance and Applications of Arenes
- Safety and Environmental Considerations of Arenes
- Future Perspectives in Arene Chemistry
What Exactly Are Arenes? Defining Aromaticity
At its core, an arene is an aromatic hydrocarbon. The most iconic and fundamental example of an arene is benzene (C₆H₆). What sets arenes apart from other cyclic hydrocarbons is a special property known as "aromaticity." This isn't just about having a pleasant smell, as the historical origin of the term might suggest; it refers to a specific electronic configuration that grants these molecules exceptional stability and unique reactivity. For a cyclic compound to be considered aromatic, and thus an arene, it must typically satisfy Hückel's Rule, which states that the molecule must be:- Cyclic and planar.
- Fully conjugated, meaning every atom in the ring must have a p-orbital.
- Contain (4n + 2) pi electrons, where 'n' is any non-negative integer (0, 1, 2, 3, etc.). For benzene, n=1, so it has (4*1 + 2) = 6 pi electrons.
The Unique Structure of Arenes: Benzene as the Blueprint
The structure of benzene is the quintessential model for understanding all arenes. For many years, chemists struggled to depict benzene accurately, proposing various structures with alternating single and double bonds. However, experiments revealed that all carbon-carbon bonds in benzene are of equal length, intermediate between a typical single and double bond. This observation led to the concept of electron delocalization. In benzene, each of the six carbon atoms is sp² hybridized, forming three sigma bonds: one with a hydrogen atom and two with adjacent carbon atoms. This creates a planar hexagonal ring. Crucially, each carbon atom also possesses an unhybridized p-orbital perpendicular to the plane of the ring. These six p-orbitals overlap laterally, both above and below the ring, creating a continuous, delocalized pi electron cloud. This delocalization of the six pi electrons (from the three double bonds) across the entire ring is what confers the extraordinary stability characteristic of arenes. This stability is often referred to as "resonance energy" or "aromatic stabilization energy." The electrons are not fixed between two specific atoms but are spread out over the entire ring, making the molecule less reactive to addition reactions (which would disrupt the aromatic system) and more prone to substitution reactions, where the aromaticity is preserved.Common Arenes and Their Derivatives
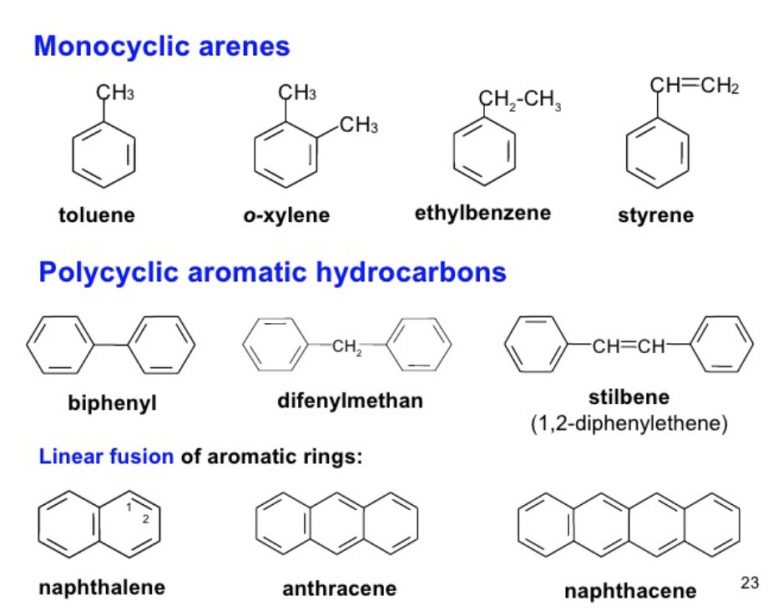
While benzene is the simplest arene, a vast family of compounds derives from it, either by replacing one or more hydrogen atoms with other functional groups or by fusing multiple benzene rings together. These derivatives exhibit a wide range of properties and applications.Alkylbenzenes and Phenyl Groups
One of the most straightforward modifications to a benzene ring is the attachment of an alkyl group. An alkylbenzene is simply a benzene ring with an alkyl group attached to it. Toluene (methylbenzene) and xylenes (dimethylbenzenes) are common examples. These compounds retain the aromaticity of the benzene ring but have slightly altered physical properties and reactivity due to the presence of the alkyl group. Alkylbenzenes are widely used as solvents and as starting materials for synthesizing other chemicals. When an aromatic ring is part of a larger molecule, it is often referred to as a functional group. Phenyl is a functional group with an aromatic ring bonded to another group. For example, in biphenyl, two phenyl groups are directly bonded. This nomenclature is crucial for accurately describing complex organic molecules. Building on this, we encounter compounds like phenol. And, phenol is a molecule that is just a phenyl bonded to a hydroxyl group (-OH). Phenol itself is an important industrial chemical, used in the production of plastics, resins, and pharmaceuticals, demonstrating the versatility of arene derivatives.Haloarenes: Aromatic Rings with Halogens
Another significant class of arene derivatives includes haloarenes, where one or more hydrogen atoms on the benzene ring are replaced by halogen atoms (fluorine, chlorine, bromine, or iodine). For instance, chlorobenzene is a common haloarene. The presence of halogens introduces interesting electronic effects on the aromatic ring. Halogens bonded to benzene ring has three lone pairs of electrons. These three electron pairs can cause resonance in benzene ring, leading to electron donation into the ring, particularly at the ortho and para positions. This resonance effect would typically suggest that halogens are activating groups, meaning they would make the ring more reactive towards electrophilic substitution. But, halogens are also highly electronegative and thus they exert a strong inductive effect, pulling electron density away from the ring through the sigma bonds. This inductive withdrawal deactivates the ring, making it less reactive than benzene towards electrophilic aromatic substitution. The interplay between the resonance (donating) and inductive (withdrawing) effects makes halogens unique substituents on arenes: they are deactivating groups overall (due to the stronger inductive effect) but are ortho/para directors (due to the resonance effect). This dual nature is a classic example of how subtle electronic interactions dictate the reactivity of arenes.Reactivity of Arenes: Electrophilic Aromatic Substitution (EAS)
Unlike alkenes, which readily undergo addition reactions, arenes primarily react via electrophilic aromatic substitution (EAS). This type of reaction involves an electrophile (an electron-deficient species) replacing a hydrogen atom on the aromatic ring, preserving the precious aromaticity. The high electron density of the delocalized pi system makes the arene ring an attractive target for electrophiles. Common EAS reactions include nitration, halogenation, Friedel-Crafts alkylation, Friedel-Crafts acylation, and sulfonation.Sulfonation: A Key Arene Reaction
Sulfonation is a particularly important EAS reaction that introduces a sulfonic acid group (-SO₃H) onto the arene ring. This reaction is crucial in the synthesis of detergents, dyes, and pharmaceuticals. The electrophile in sulfonation is typically sulfur trioxide (SO₃), which can be generated in various ways depending on the reaction conditions. In concentrated SO₃ or oleum, two molecules of SO₃ form a transition state with the arene, acting as the electrophilic species. This reaction is often reversible, allowing for the removal of the sulfonic acid group under specific conditions (e.g., heating with dilute acid), which is a useful synthetic tool. The mechanism of sulfonation can be complex, involving different species depending on the acid concentration. For instance, when carried out in sulfuric acid, it is a concerted mechanism where the electrophile and the proton removal occur in a somewhat synchronized fashion, or in sulfuric acid, the termolecular complex involves the interaction of the arene with two molecules of sulfuric acid, one acting as a proton donor and the other as a proton acceptor, facilitating the electrophilic attack by SO₃. This intricate mechanistic detail highlights the depth of understanding required in organic chemistry.Directing Effects and Arene Substitution Patterns
When a benzene ring already has a substituent, the position at which a second substituent enters the ring is not random. The existing substituent influences the reactivity and regioselectivity (where the new group attaches). This is known as the directing effect. Substituents are broadly classified as either ortho/para directors or meta directors.- Ortho/Para Directors: These groups direct incoming electrophiles to the ortho (adjacent) and para (opposite) positions relative to themselves. They are typically electron-donating groups (e.g., -OH, -NH₂, -CH₃, halogens). They also activate the ring, making it more reactive than benzene.
- Meta Directors: These groups direct incoming electrophiles to the meta (one carbon away) position. They are typically electron-withdrawing groups (e.g., -NO₂, -COOH, -SO₃H). They also deactivate the ring, making it less reactive than benzene.
Arene Spectroscopy: Unveiling Molecular Fingerprints
Identifying and characterizing arenes, like any organic compound, relies heavily on spectroscopic techniques. These methods provide unique "fingerprints" that reveal details about the molecule's structure.- Nuclear Magnetic Resonance (NMR) Spectroscopy: Proton NMR (¹H NMR) is particularly useful for arenes. Aromatic protons typically resonate in a distinct region (around 6.5-8.5 ppm), shifted downfield due to the anisotropic effect of the ring current. The splitting patterns of these signals provide information about the number of adjacent protons, helping to determine the substitution pattern (e.g., ortho, meta, para). Carbon-13 NMR (¹³C NMR) provides signals for each unique carbon atom in the ring. I have seen lots of people labeling both signals in this region, indicating the presence of aromatic carbons, which are distinct from aliphatic carbons.
- Infrared (IR) Spectroscopy: Arenes show characteristic C-H stretching vibrations around 3030 cm⁻¹ (above 3000 cm⁻¹) and C=C stretching vibrations within the ring around 1600 cm⁻¹ and 1500 cm⁻¹. Out-of-plane C-H bending vibrations in the fingerprint region (below 900 cm⁻¹) can also indicate the substitution pattern (monosubstituted, ortho-, meta-, or para-disubstituted).
- Mass Spectrometry (MS): MS provides information about the molecular weight and fragmentation patterns. Arenes often show a strong molecular ion peak due to their stability. Fragmentation patterns can reveal the nature of substituents.
- Ultraviolet-Visible (UV-Vis) Spectroscopy: Arenes typically exhibit characteristic absorption bands in the UV region due to their conjugated pi electron systems, which can be useful for quantitative analysis or detecting the presence of aromatic rings.
Industrial Importance and Applications of Arenes
The industrial significance of arenes cannot be overstated. They are indispensable building blocks and intermediates in a vast array of manufacturing processes:- Polymers and Plastics: Benzene and its derivatives are precursors to many polymers. Styrene (phenylethene), derived from benzene, is polymerized to produce polystyrene, a widely used plastic. Phenol is crucial for making phenolic resins (Bakelite) and polycarbonates.
- Pharmaceuticals: Many drugs contain aromatic rings as core structures. Aspirin (acetylsalicylic acid), paracetamol (acetaminophen), and numerous antibiotics and antidepressants are examples where arene moieties are integral to their therapeutic activity. The specific arrangement of functional groups on the aromatic ring often dictates the drug's efficacy and selectivity.
- Dyes and Pigments: The extended conjugated systems of many dyes and pigments incorporate multiple aromatic rings, often linked by nitrogen-containing bridges (azo dyes). The delocalized electrons within these arene-based structures are responsible for absorbing specific wavelengths of light, giving them their vibrant colors.
- Solvents: Toluene, xylene, and benzene (though less used now due to toxicity) are excellent organic solvents for a wide range of nonpolar and moderately polar compounds. They are used in paints, coatings, adhesives, and chemical reactions.
- Agrochemicals: Many herbicides, insecticides, and fungicides are derivatives of arenes, designed to target specific biological pathways in pests or weeds.
- Fuels: Benzene, toluene, and xylenes (BTX) are components of gasoline, increasing its octane rating. While benzene content in gasoline is now highly regulated due to health concerns, other arenes remain important.
Safety and Environmental Considerations of Arenes
While incredibly useful, arenes also pose significant safety and environmental challenges, particularly benzene. Benzene is a known human carcinogen, primarily linked to leukemia. Exposure can occur through inhalation or skin contact. Due to its toxicity, the use of benzene as a solvent has been severely restricted, and efforts are continuously made to minimize human exposure in industrial settings. Other arenes, while generally less toxic than benzene, still require careful handling. Many are volatile and flammable, posing fire and explosion hazards. Their persistence in the environment, particularly if released into water or soil, can lead to long-term contamination issues. For example, industrial spills of alkylbenzenes can affect groundwater and ecosystems. The chemical industry is increasingly focused on "green chemistry" principles, aiming to develop more sustainable and safer methods for producing and utilizing arenes. This includes designing less hazardous synthetic routes, developing catalysts that reduce waste, and exploring bio-based alternatives where feasible. Responsible management of arene production, use, and disposal is paramount to mitigate their potential negative impacts on human health and the environment.Future Perspectives in Arene Chemistry
The field of arene chemistry continues to evolve, driven by the demand for new materials, more efficient synthetic methods, and sustainable practices. Researchers are exploring several exciting avenues:- Catalysis for Selective Arene Functionalization: Developing new catalysts that can selectively functionalize arenes at specific positions without requiring pre-functionalization (e.g., C-H activation) is a major area of research. This could simplify synthetic routes and reduce waste.
- Sustainable Arene Synthesis: Exploring bio-based feedstocks for arene production, moving away from fossil fuels, and developing energy-efficient synthesis methods are crucial for a sustainable future. This includes using renewable resources and benign solvents.
- Novel Arene-Based Materials: The unique electronic properties of arenes make them ideal for advanced materials, including organic semiconductors, light-emitting diodes (OLEDs), and components for solar cells. Research into novel fused arene systems and conjugated polymers continues to push the boundaries of materials science.
- Medicinal Chemistry Advancements: The design of new drugs often involves modifying arene scaffolds to improve efficacy, reduce side effects, or overcome drug resistance. Understanding the subtle electronic and steric effects of substituents on arene rings is key to rational drug design. For instance, in drug discovery, a common challenge is to achieve high selectivity in reactions. Or in simpler terms, does the rearrangement exclusively form only one product or does it form some small amount of undesirable byproducts? This question of selectivity is paramount when synthesizing complex pharmaceutical ingredients, where even trace impurities can be detrimental.
Conclusion
Arenes stand as a cornerstone of organic chemistry, their unique aromaticity providing a foundation for countless molecules that underpin our modern world. From the fundamental stability of benzene to the complex reactivity of its substituted derivatives, these compounds demonstrate the elegant interplay of structure and function in chemistry. We've explored their defining characteristics, delved into their common forms like alkylbenzenes and haloarenes, and uncovered the intricate mechanisms of reactions like electrophilic aromatic substitution, which allows for the precise construction of complex molecules. The pervasive presence of arenes in pharmaceuticals, polymers, and industrial chemicals underscores their immense utility, while ongoing research into their sustainable synthesis and novel applications promises an even more impactful future. The journey into arenes reveals not just the beauty of molecular design but also the critical importance of understanding their properties for both progress and safety. We encourage you to delve deeper into the fascinating world of organic chemistry. What aspects of arenes do you find most intriguing? Share your thoughts and questions in the comments below, or explore other articles on our site to continue your chemical exploration!Related Resources:


Detail Author:
- Name : Martine Zulauf Sr.
- Username : littel.juston
- Email : rohan.faye@gmail.com
- Birthdate : 1995-02-15
- Address : 299 Eloisa Lake Apt. 705 Cassieshire, HI 93218
- Phone : 1-920-392-1903
- Company : Kozey, Glover and Kassulke
- Job : Computer Systems Analyst
- Bio : Nihil voluptatem non est ex voluptatum. Explicabo ex ea et quam itaque optio. Tempora quod omnis sit pariatur tempore.
Socials
twitter:
- url : https://twitter.com/maida1136
- username : maida1136
- bio : Aut ullam commodi cum. Impedit distinctio et voluptatem. Quam officia eligendi optio a quia sapiente.
- followers : 2533
- following : 2054
facebook:
- url : https://facebook.com/maida.carroll
- username : maida.carroll
- bio : Consequatur in rem possimus dolorum sed.
- followers : 746
- following : 85
instagram:
- url : https://instagram.com/maida_carroll
- username : maida_carroll
- bio : Voluptatibus vero tempore occaecati perferendis. Quo ipsam modi culpa enim corrupti.
- followers : 2457
- following : 625
tiktok:
- url : https://tiktok.com/@mcarroll
- username : mcarroll
- bio : Sunt quasi aut accusamus voluptatem tempora ut qui.
- followers : 5345
- following : 583
linkedin:
- url : https://linkedin.com/in/mcarroll
- username : mcarroll
- bio : Dolorem sed unde quidem.
- followers : 3467
- following : 1881